After studying this chapter students will be able to:
• describe a simple model for the atom to include protons, neutrons and
electrons.
• determine the number of protons, neutrons and nucleons it contains for the
specification of a nucleus in the form 2XA.
• explain that an element can exist in various isotopic forms each with a
different number of neutrons.
• explain the use of mass spectrograph to demonstrate the existence of
isotopes and to measure their relative abundance.
• define the terms unified mass scale, mass defect and calculate binding
energy using Einstein's equation.
• illustrate graphically the variation of binding energy per nucleon with the
mass number.
• explain the relevance of binding energy per nucleon to nuclear fusion and
to nuclear fission.
• identify that some nuclei are unstable, give out radiation to get rid of
excess energy and are said to be radioactive.
• describe that an element may change into another element when
radioactivity occurs.
• identify the spontaneous and random nature of nuclear decay.
• describe the term half-life and solve problems using the equation
λ=0.693/T 1/2
• determine the release of energy from different nuclear reactions.
explain that atomic number and mass number conserve in nuclear
reactions.
describe energy and mass conservation in simple reactions and in
radioactive decay.
describe the phenomena of nuclear fission and fusion.
describe the fission chain reaction.
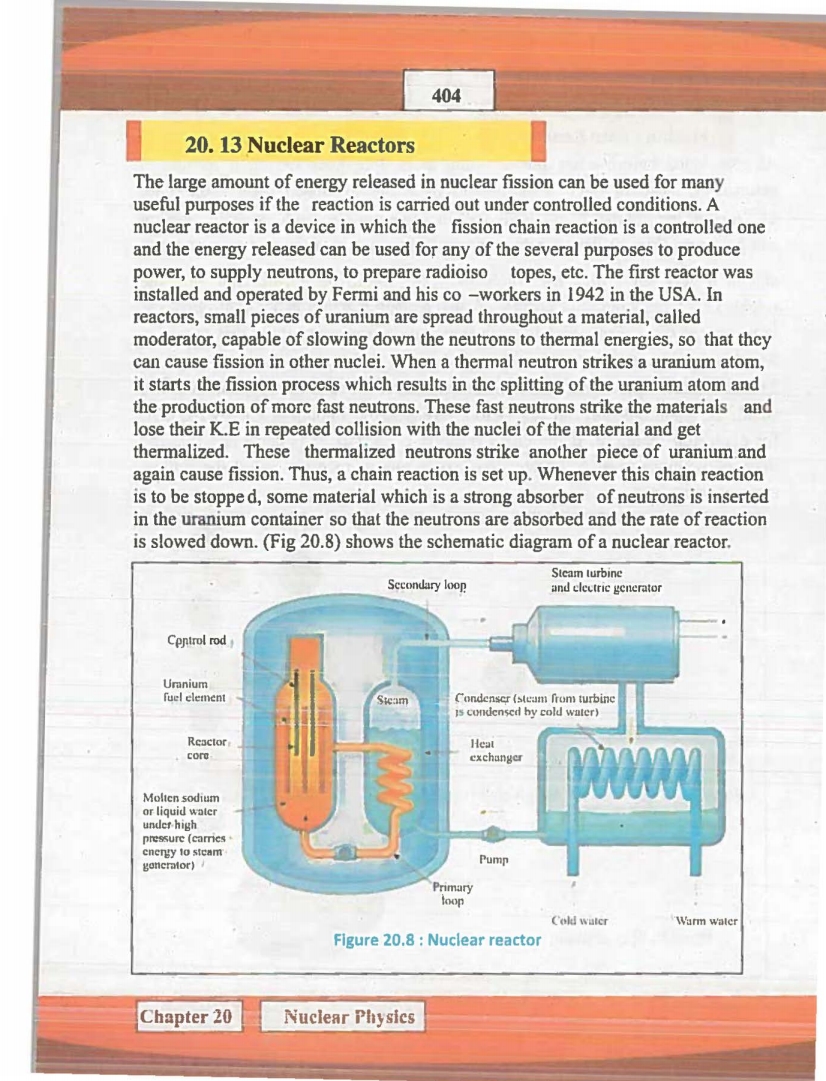
• describe the function of various components of a nuclear reactor.
• describe the interaction of nuclear radiation with matter.
a describe the use of Geiger Muller counter and solid-state detectors to
detect the radiations.
describe the basic forces of nature.
Ga describe the key features and components of the standard model of matter
including hadrons, leptons and quarks.

























































Post a Comment